Abstract
Introduction
Hemarthroses are serious adverse experiences for patients with hemophilia A that lead to the development of hemophilic arthropathy. Regular prophylaxis with factor VIII (FVIII) can reduce hemophilic arthropathy, but there is a need for biomarkers that predict joint deterioration and guide intensified treatment. Collagen is a major structural component of the highly vascular synovium, in which type 4 collagen is the main constituent of the basement membrane and essential for vascular mechanical stability. Type 4 collagen levels are elevated at baseline and in response to hemarthrosis in hemophilic rats (Manon-Jensen et al. JTH 2016;14:2419-29) and are associated with painful joint bleeds in patients with hemophilia (Gopal et al. JTH 2021;19:1200-11). In patients with rheumatoid arthritis, type 4 collagen is associated with progression of joint destruction (Gudmann et al. Clin Exp Rheumatol 2018;36:829-35).
Aim
The aim of this study was to examine the relationship of collagen biomarkers of joint health and factor replacement therapy targeting two different FVIII trough levels in patients with severe hemophilia A.
Methods
Design and primary outcomes of the PROPEL study (NCT0285960) have been reported previously (Klamroth et al. Blood 2021;137:1818-27). Patients with hemophilia A who had either completed a previous rurioctocog alfa pegol study or were naive to rurioctocog alfa pegol were randomized to 12 months' pharmacokinetic-guided prophylaxis targeting FVIII troughs of 1-3% or 8-12%.
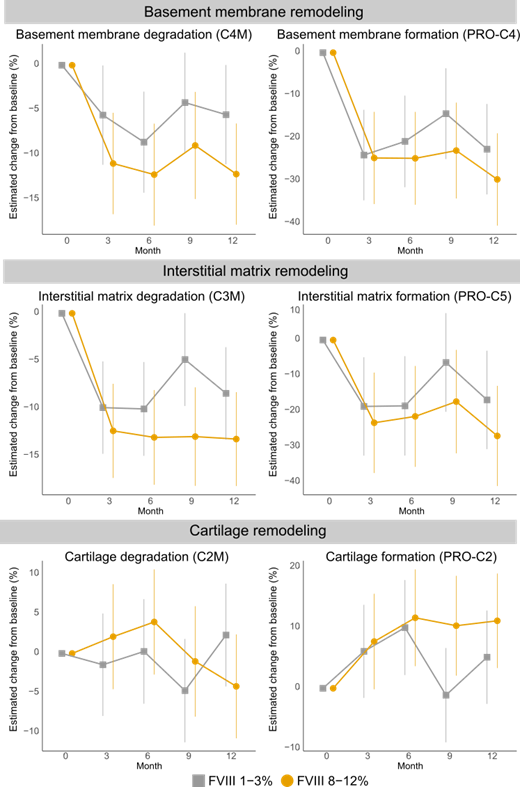
Biomarkers reflecting basement membrane remodeling (collagen type 4 formation PRO-C4 and degradation C4M), interstitial matrix remodeling (collagen type 5 formation PRO-C5 and collagen type 3 degradation C3M), and cartilage remodeling (collagen type 2 formation PRO-C2 and degradation C2M) were measured blinded in serum by enzyme-linked immunosorbent assay in a CAP-certified laboratory.
Longitudinal biomarker data for each patient were collected at 5 time points (baseline and months 3, 6, 9, and 12), and linear mixed-effect models were used to analyze (logarithm-based) percentage change in serological biomarker levels from baseline to study completion. The models included time from baseline (months), treatment arm interaction, and baseline levels of the biomarker as covariates, with patient as a random effect.
Results
Biomarkers were measured in 98 patients. Fifty patients were randomized to the 1-3% and 48 to the 8-12% FVIII trough level treatment arms. Before normalization, C4M and PRO-C4, were elevated by an average of 38% compared with healthy controls.
C4M and PRO-C4 significantly decreased after 3 months, by 5.6% and 24.0% in the 1-3% FVIII trough level arm (p= 0.049 and p<0.0001) and 11.0% and 24.7% in the 8-12% FVIII trough level arm (p=0.0002 and p<0.0001), respectively. The same was observed for C3M and PRO-C5. C3M and PRO-C5 significantly decreased after 3 months, by 9.9% and 18.7% in the 1-3% arm (p=0.0001 and p=0.009) and 11.0% and 23.3% in the 8-12% arm (p=0.0002 and p=0.001), respectively (Figure). A significant increase was seen for the cartilage formation marker PRO-C2, with 10.0% and 11.7% increase after 6 months in the 1-3% arm and 8-12% arm (p=0.01 and p=0.005), respectively. No significant difference was observed for the cartilage degradation biomarker C2M.
When stratified by prior treatment, significant changes were demonstrated in on-demand patients, with a 17.9% difference for C4M between FVIII 1-3% and FVIII 8-12% after 6 months (p=0.02). The same was seen for C3M, with a 15.1% difference between FVIII 1-3% and FVIII 8-12% after 6 months (p=0.03). No differences were seen in patients who were on FVIII prophylaxis prior to study entry.
Conclusion
Continuous FVIII prophylaxis led to a dose-dependent decrease in remodeling of the basement membrane and interstitial matrix as well as an increase in cartilage formation in both treatment arms, especially when stratified by prior treatment regimen. Together, the data suggest that FVIII prophylaxis leads to a normalization of joint remodeling. This is further supported by primary data from PROPEL, which demonstrated that targeting FVIII 8-12% vs 1-3% results in a lower annualized bleeding rate. This novel biomarker data is the first to demonstrate the importance of FVIII prophylaxis for joint health in patients with hemophilia A from a randomized clinical trial.
Manon-Jensen: Nordic Bioscience: Current Employment, Current equity holder in publicly-traded company. Tangada: Takeda Development Center Americas, Inc: Current Employment; Takeda: Current equity holder in publicly-traded company. Bager: Nordic Bioscience: Current Employment, Current equity holder in publicly-traded company. Chowdary: CSL Behring: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; SOBI: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Boehringer Ingelheim: Honoraria; Chugai: Honoraria; Roche: Honoraria; Sanofi: Honoraria; Spark: Honoraria; Novo Nordisk: Honoraria, Research Funding; Freeline: Honoraria, Research Funding. Klamroth: Octapharma: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Roche/Cugai: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Biotest: Consultancy, Other: Travel support, Speakers Bureau; BioMarin: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Shire (a Takeda company): Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Pfizer: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Bayer: Consultancy, Other: Clinical trial investigator, Travel support, Research Funding, Speakers Bureau; Sanofi: Consultancy, Other: Clinical trial investigator, Travel support, Speakers Bureau; Sobi: Consultancy, Other, Speakers Bureau; Uniqure: Consultancy, Other; LEO: Other, Research Funding, Speakers Bureau; Daiichi Sankyo: Other, Speakers Bureau; Grifols: Speakers Bureau. von Drygalski: Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Research Funding; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Hematherix, Inc: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: Super FVa; CSL Behring: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Biomarin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; uniQure: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Windyga: Alnylam Pharmaceuticals: Research Funding; Novo Nordisk: Research Funding, Speakers Bureau; Octapharma: Research Funding, Speakers Bureau; Rigel: Research Funding; Roche: Research Funding, Speakers Bureau; Sanofi/Genzyme: Research Funding, Speakers Bureau; Shire/takeda: Research Funding, Speakers Bureau; Sobi: Research Funding, Speakers Bureau; Alexion: Speakers Bureau; CSL Behring: Speakers Bureau; Werfen: Speakers Bureau. Frederiksen: Nordic Bioscience: Current Employment. Engl: Baxaltha innovations GmbH, a Takeda company: Current Employment; Takeda: Current equity holder in publicly-traded company. Ewenstein: Takeda: Current equity holder in publicly-traded company; Takeda Development Center Americas, Inc.: Current Employment. Karsdal: Nordic Bioscience: Current Employment, Current equity holder in publicly-traded company.